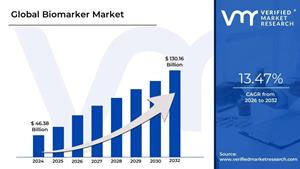
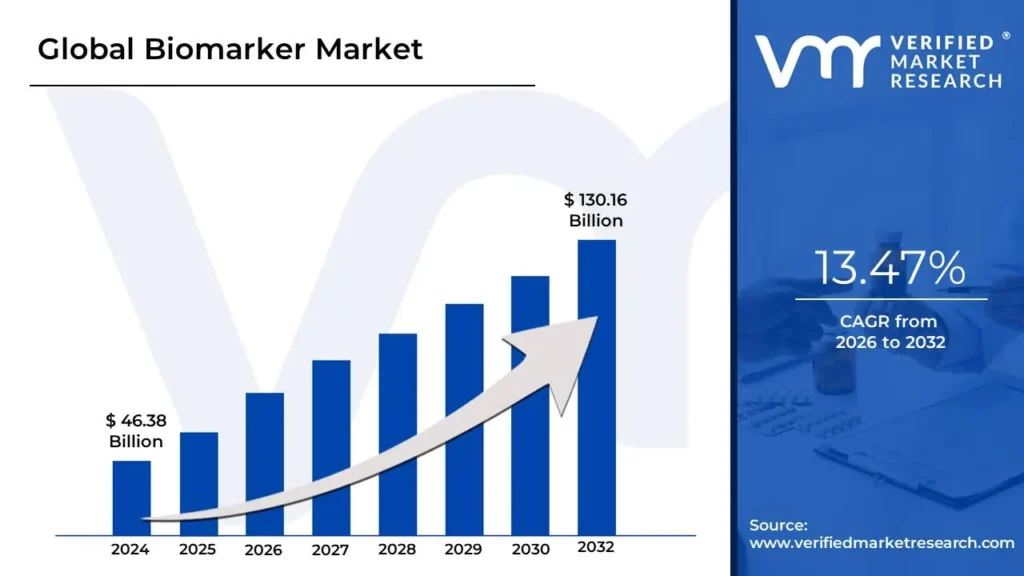
Biomarker Market is expected to generate a revenue of USD 130.16 Billion by 2032, Globally, at 13.77% CAGR: Verified Market Research®
The biomarker market is poised for exponential growth driven by the personalized medicine revolution, advanced diagnostics, and robust R&D ecosystems. However, market participants must navigate regulatory complexities, high validation costs, and reimbursement gaps to succeed. North America's dominance highlights the importance of infrastructure, policy support, and innovation alignment. Companies seeking market entry or expansion should focus on strategic collaborations, regulatory preparedness, and demonstrating clinical-economic value to gain competitive advantage and long-term sustainability in this evolving space.
Lewes, Delaware, June 04, 2025 (GLOBE NEWSWIRE) -- The Global Biomarker Market Size is projected to grow at a CAGR of 13.77% from 2026 to 2032, according to a new report published by Verified Market Research®. The report reveals that the market was valued at USD 46.38 Billion in 2024 and is expected to reach USD 130.16 Billion by the end of the forecast period.
The global biomarker market is witnessing accelerated growth, driven by the need for early disease detection, precision therapeutics, and technological innovations in genomics and diagnostics.
Key Highlights of the Report:
- Market Size & Forecast – In-depth analysis of current value and future projections
- Segment Analysis – Detailed study across Type, Product, Disease Indication, and Application.
- Regional Insights – Comprehensive coverage of North America, Europe, Asia-Pacific, and more
- Competitive Landscape – Profiles of top players and their strategic initiatives
- Regulatory Impact – Assessment of global and regional compliance frameworks
- Technological Innovations Mapped: Coverage of latest advancements in omics technologies and AI-based biomarker discovery.
-
Challenges and Risk Assessment: Evaluates ethical debates, off-target effects, and regulatory complexities.
Why This Report Matters:
This report offers actionable insights into market dynamics, helping stakeholders align their strategic decisions with emerging trends. It supports data-driven planning, risk assessment, and opportunity evaluation in the evolving biomarker landscape.
Who You Should Read This Report:
- Pharmaceutical & Biotechnology Executives
- Diagnostic Tool Manufacturers
- Clinical Researchers & Healthcare Analysts
- Venture Capitalists & Institutional Investors
- Policy Makers & Regulatory Professionals
 For more information or to purchase the report, please contact us at: https://www.verifiedmarketresearch.com/download-sample/?rid=62283
For more information or to purchase the report, please contact us at: https://www.verifiedmarketresearch.com/download-sample/?rid=62283
Browse in-depth TOC on “Global Biomarker Market Size”
202 - Pages
126 – Tables
37 – Figures
Report Scope
| REPORT ATTRIBUTES | DETAILS |
| STUDY PERIOD | 2021-2032 |
| BASE YEAR | 2024 |
| FORECAST PERIOD | 2026-2032 |
| HISTORICAL PERIOD | 2021-2023 |
| KEY COMPANIES PROFILED | Qiagen N.V., Perkinelmer Inc., Merck Millipore, Bio-Rad Laboratories Inc., Enzo Biochem Inc., EKF Diagnostics Holdings Inc., Meso Scale Diagnostics LLC. |
| UNIT | Value (USD Billion) |
| SEGMENTS COVERED | By Type, By Product, By Disease Indication, By Application And By Geography |
| CUSTOMIZATION SCOPE | Free report customization (equivalent up to 4 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope |
Global Biomarker Market Overview
Market Driver
Surge in Demand for Precision Medicine and Targeted Therapies: Biomarkers are now at the forefront of modern medicine due to the paradigm shift away from universal treatment procedures and toward individualized therapy. For example, biomarkers aid in the identification of genetic abnormalities in oncology, enabling physicians to prescribe highly specific medications such as HER2-targeted therapy for breast cancer or EGFR inhibitors for lung cancer. Biomarkers are making it possible for more efficient, patient-centered interventions as global healthcare systems place a higher priority on individualized results and fewer side effects. Their growing application in pharmacogenomics to forecast side effects and drug response is expediting treatment, lowering hospitalization rates, and maximizing the use of medical resources. Furthermore, biomarkers play a crucial role in clinical trial patient stratification, trial efficiency, and regulatory approval speed, all of which increase demand for them throughout the therapeutic spectrum.
Expanding Investments in R&D and Biomarker Discovery: The market for biomarkers is seeing a sharp increase in funding from the public and private sectors. To lower attrition rates in drug development and improve the likelihood of success in Phase II/III trials, pharmaceutical companies, diagnostics companies, and contract research organizations are making significant investments in biomarker identification. Early-stage research and innovation are being accelerated by government programs such as the EU's Horizon Europe financing and the U.S. NIH's Biomarkers Consortium. Furthermore, translational research is being promoted via academic-industry collaborations, which are advancing laboratory findings toward clinical implementation. Large genomic databases and improvements in omics technologies are supporting this ecosystem even more by facilitating the high-throughput identification and validation of novel biomarkers across therapeutic domains like metabolic disorders, neurodegenerative diseases, and autoimmune diseases.
Technological Advancements in Genomics and Proteomics Platforms: The development of molecular biology technologies has greatly sped up the process of finding and using biomarkers. The sensitivity, specificity, and speed of biomarker identification have been improved by next-generation sequencing (NGS), single-cell RNA sequencing, proteomic mapping, and CRISPR-based technologies. Large molecular datasets may now be interpreted in real time using AI-powered analytics and big data algorithms, which provide useful biomarkers for clinical decision-making. A move toward multiplex biomarker panels and companion diagnostics, which provide thorough insights into disease mechanisms, is being made possible by such advancements. Additionally, early illness identification is becoming more accessible because to point-of-care biomarker diagnostics, which are backed by microfluidics and biosensor technologies, especially in environments with low resources. Because of these technical advancements, biomarkers are becoming more useful in complex illness management for predictive, prognostic, and monitoring purposes rather than only diagnostic ones.
 To Purchase a Comprehensive Report Analysis: https://www.verifiedmarketresearch.com/select-licence/?rid=62283
To Purchase a Comprehensive Report Analysis: https://www.verifiedmarketresearch.com/select-licence/?rid=62283
Market Restraint
High Costs Associated with Biomarker Validation and Clinical Trials: From discovery to clinical use, biomarker development is a demanding and resource-intensive procedure. In contrast to traditional diagnoses, biomarkers need to be validated in a variety of groups in order to be clinically relevant and reproducible. This involves high-throughput screening, sophisticated apparatus, and longitudinal cohort studies, all of which increase development costs. Before biomarker validation reaches the commercialization stage, it typically takes years and millions of dollars. Furthermore, these exorbitant expenses continue throughout the clinical trial stage, where biomarkers are employed for patient stratification or incorporated into adaptive trial designs. Smaller research businesses and startups frequently lack the financial resources to maintain this drawn-out pipeline, which results in significant attrition and lost commercial potential. As a result, this financial barrier prevents new competitors from entering the market and postpones the broad use of innovative biomarkers in clinical settings.
Regulatory Complexities and Delayed Approvals: The fragmented and changing regulatory environment is one of the main obstacles impeding the growth of the biomarker industry. Strict rules for biomarker qualification and use in clinical trials and diagnostics are set by organizations such as the FDA and EMA. Businesses must show utility across certain patient populations in addition to clinical relevance and analytical validity, frequently through costly and time-consuming experiments. International product releases are made more difficult and the regulatory cost is increased by the lack of a worldwide standardized biomarker approval process. Time-to-market is further prolonged by the fact that biomarkers categorized as companion diagnostics or laboratory-developed tests (LDTs) frequently need independent and parallel approval processes. Innovation cycles are slowed and private investment is discouraged by these regulatory concerns, particularly for biomarkers with strong scientific potential but little immediate economic value.
Limited Reimbursement Frameworks in Biomarker Diagnostics: Even with their clinical utility, biomarker-based tests have a difficult time getting paid, especially in healthcare systems with tight budgets. Before providing coverage, payers and insurers require strong proof of clinical utility and health-economic benefits, which many biomarker tests—particularly more recent or intricate panels—fail to deliver in the early phases. Reimbursement for molecular diagnostics is still inconsistent or non-existent in many regions, including parts of Asia, Latin America, and Eastern Europe. Disparities in test availability and uptake result from the significant differences in reimbursement rules between public and private insurers, even in industrialized nations. Real-world use is limited by the out-of-pocket expense, which discourages patients and providers from choosing sophisticated biomarker testing. This reduces diagnostic companies' financial incentives and stymies innovation, continuing the cycle in which even validated biomarkers find it difficult to acquire momentum in the market because of reimbursement gaps.
Geographical Dominance
North America currently dominates the biomarker industry, owing to its strong research infrastructure, early embrace of precision medicine, and well-established healthcare payment schemes. High R&D expenditures, advantageous regulatory frameworks, and substantial partnerships between academic institutions and biotech companies are all advantages for the area. Furthermore, the increasing incidence of chronic illnesses and the presence of significant pharmaceutical companies hasten the acceptance of biomarkers and market expansion.
Key Players
The “Global Biomarker Market” study report will provide a valuable insight with an emphasis on the global market. The major players in the market are Qiagen N.V., Perkinelmer Inc., Merck Millipore, Bio-Rad Laboratories Inc., Enzo Biochem Inc., EKF Diagnostics Holdings Inc., Meso Scale Diagnostics LLC.
Biomarker Market Segment Analysis
Based on the research, Verified Market Research has segmented the global market into Type, Product, Disease Indication, Application, and Geography.
-
Biomarker Market, by Type
- Safety Biomarker
- Efficacy Biomarker
- Predictive Biomarker
- Surrogate Biomarker
- Pharmacodynamic Biomarker
- Prognostic Biomarker
- Validation Biomarker
-
Biomarker Market, by Product
- Consumables
- Services
- Software
-
Biomarker Market, by Disease Indication
- Cancer
- Cardiovascular Disorders
- Neurological Disorders
- Immunological Disorders
-
Biomarker Market, by Application
- Diagnostics
- Drug Discovery and Development
- Personalized Medicine
- Disease Risk Assessment
-
Biomarker Market, by Geography
-
North America
- U.S
- Canada
- Mexico
-
Europe
- Germany
- France
- U.K
- Rest of Europe
-
Asia Pacific
- China
- Japan
- India
- Rest of Asia Pacific
-
ROW
- Middle East & Africa
- Latin America
-
North America
Browse Related Reports:
Global Cancer Biomarkers Market Size By Cancer Type (Breast Cancer, Prostate Cancer), By Biomarker-Type (Protein Biomarkers, Genetic Biomarkers), By Profiling Technology (OMICS Technology, Imaging Technology), By Application (Drug Discovery And Development, Diagnostics), By Geography, And Forecast
Global Non-Alcoholic Steatohepatitis Biomarkers Market Size By Type of Biomarker (Serum Biomarkers, Hepatic Fibrosis Biomarkers, Apoptosis Biomarkers), By End User (Pharmaceutical Companies and Cros, Research Institutes and Academics), By Geography, And Forecast
Global Cardiac Biomarker Diagnostic Kits Market Size By Indication (Myocardial Infarction, Congestive Heart Failure, Acute Coronary Syndrome), By Biomarker (Troponin, Bnp, Myoglobin), By End-Use (Hospital, Specialty Clinics), By Geography, And Forecast
Global Exosome Research Market Size By Product & Service (Kits & Reagents, Instruments, Services), By Indication (Cancer, Neurodegenerative Diseases, Cardiovascular Diseases, Infectious Diseases), By Application (Biomarkers, Vaccine Development, Tissue Regeneration), By End-User (Academic & Research Institutes, Pharmaceutical & Biotechnology Companies, Hospitals & Clinical Laboratories), By Geography, And Forecast
Top 7 Biomaterial Companies modernizing treatment and rehabilitation process
Visualize Biomarker Market using Verified Market Intelligence -:
Verified Market Intelligence is our BI Enabled Platform for narrative storytelling in this market. VMI offers in-depth forecasted trends and accurate Insights on over 20,000+ emerging & niche markets, helping you make critical revenue-impacting decisions for a brilliant future.
VMI provides a holistic overview and global competitive landscape with respect to Region, Country, Segment, and Key players of your market. Present your Market Report & findings with an inbuilt presentation feature saving over 70% of your time and resources for Investor, Sales & Marketing, R&D, and Product Development pitches. VMI enables data delivery In Excel and Interactive PDF formats with over 15+ Key Market Indicators for your market.
About Us
Verified Market Research® stands at the forefront as a global leader in Research and Consulting, offering unparalleled analytical research solutions that empower organizations with the insights needed for critical business decisions. Celebrating 10+ years of service, VMR has been instrumental in providing founders and companies with precise, up-to-date research data.
With a team of 500+ Analysts and subject matter experts, VMR leverages internationally recognized research methodologies for data collection and analyses, covering over 15,000 high impact and niche markets. This robust team ensures data integrity and offers insights that are both informative and actionable, tailored to the strategic needs of businesses across various industries.
VMR's domain expertise is recognized across 14 key industries, including Semiconductor & Electronics, Healthcare & Pharmaceuticals, Energy, Technology, Automobiles, Defense, Mining, Manufacturing, Retail, and Agriculture & Food. In-depth market analysis cover over 52 countries, with advanced data collection methods and sophisticated research techniques being utilized. This approach allows for actionable insights to be furnished by seasoned analysts, equipping clients with the essential knowledge necessary for critical revenue decisions across these varied and vital industries.
Verified Market Research® is also a member of ESOMAR, an organization renowned for setting the benchmark in ethical and professional standards in market research. This affiliation highlights VMR's dedication to conducting research with integrity and reliability, ensuring that the insights offered are not only valuable but also ethically sourced and respected worldwide.
Attachment

Mr. Edwyne Fernandes Verified Market Research® US: +1 (650)-781-4080 US Toll Free: +1 (800)-782-1768 Email: sales@verifiedmarketresearch.com Web: https://www.verifiedmarketresearch.com/ Follow Us: LinkedIn | Twitter | Threads | Instagram | Facebook SOURCE – Verified Market Research®
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.